Research with
International Bowel Ultrasound Group
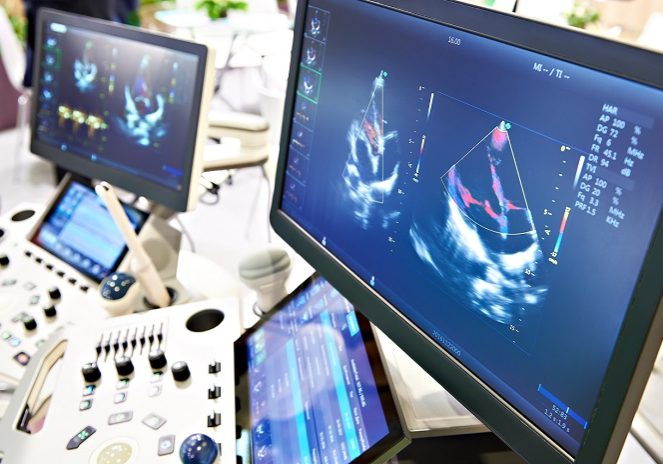
Treatment targets for IBD have evolved beyond clinical symptoms and remission. The recognition of the importance of healing seen on cross-sectional imaging modalities, including ultrasound is growing, predictive of improved outcomes for patients. IUS is cost-effective, well tolerated by patients and thus easily repeatable. This creates a perfect monitoring tool, yet access to this important innovation is limited in many parts of the world. Increasing the scientific evidence foundation to support the use of IUS is imperative to increase uptake.
Guideline
Intestinal Ultrasound Standardized
Topics & Key Questions
The aim of this publication, is to provide standardized guidance to expert clinicians regarding image acquisition during intestinal ultrasound assessment with the intent of centralized reading (for example, for the purpose of evaluation during clinical trials). The purpose is to best standardized image acquisition, to improve consistency in interpretation.
- Image Acquisition Guidelines
- Bowel wall thickness
- Mesenteric, inflammatory fat
- Echostratification
- Color Doppler Imaging
How we work
Services
Study centres
We offer research consulting and collaboration within the field of inflammatory bowel diseases, mainly Crohn's Disease and Ulcerative Colitis. We have great expertise in designing studies, defining endpoints and have a large professional network.
We need you!
We are looking for new study centers. Be a part of the IBUS association!
Requirement:
Qualitified staff,
minimum number of Sonography,
Email: jc@bowel-ultrasound.org
New Centers to join soon!
ECCO-IBUS RESEARCH GRANT
2024
The International Bowel Ultrasound Group (IBUS) and the European Crohn’s and Colitis Organization invites all interested member investigators to submit clinical research proposals to be considered for inaugural funding support jointly provided through IBUS and ECCO.
The clinical research fund has been increased to 40,000 EUR and will be awarded to the successful investigator. Applicants must be IBUS members at the time of submission. IBUS aims to support clinical excellence in the investigation of utility of intestinal ultrasound used in the assessment of patients with inflammatory bowel disease.

KEY DATES
March
|
Submissions open
|
November
|
Application deadline |
Early 2025 |
Successful grant funding announcement, notification will be sent to all applicants |
February
|
Official hand-over of award certificate during IBUS members' meeting at ECCO congress in Berlin, Germany. |
Application overview
Selection criteria
Requirements
The protocol should be no more than 5 pages in length and include:
- Scientific Aim – clear description of the objectives of the project
- Background/ significance – justification of the importance of the project
- Methods/ research plan – a detailed account of timeline, methodology and outputs
- Budget – accounting of funds
- References
In addition, a curriculum vitae is required.
2023 IBUS-ECCO Grant Winners
#1 - awarded 20,000 euros
- Dr. Gabriele Dragoni
Ultrasound radiomics to assess intestinal inflammation and fibrosis in stricturing Crohn's disease: a pilot study
2022 IBUS-ECCO Grant Winners
#1 - awarded 20,000 euros
- Dr. Shintaro Sagami and team at Kitasato University Kitasato Institute Hospital, Tokyo, Japan
Is advanced transperineal ultrasonography in ulcerative colitis useful for predicting the response to induction therapy?
2021 IBUS-ECCO Grant Winners - Congratulations!
#1 - awarded 20,000 euros
- Dr. Jennifer DeBruyn and team from Canada
Intestinal ultrasound monitoring of response to biologics in pediatric Crohn's disease
#2 and #3 – each award 10,000 euros
- Dr. Maarten Pruijt and team from the Netherlands
PredIctioN and cloSe monItorinG of postoperative recurrence by intestinal ultrasound after ileocecal resection in CroHn's disease patienTs: the INSIGHT study
- Dr. Bram Verstockt and team from Belgium
Intestinal bowel ultrasound in acute severe ulcerative colitis, the TABASCO study
2020 IBUS-ECCO Grant Winners
#1 - awarded 20,000 euros
- Dr. Kunal Thacker and team from Australia
Diagnostic accuracy of point of care intestinal ultrasound compared to MRE and/or colonoscopy in paediatric inflammatory bowel disease
#2 and #3 – each award 10,000 euros
- Dr. Cathy Lu and team from Canada
Development of an expert consensus to standardize intestinal ultrasound definitions and reporting for Crohn’s disease non-perianal complications
- Dr. Terracciano Fulvia and team from Italy
Transperineal US in the follow up of patients with perianal Crohn’s disease: compared with MRI
2019 IBUS-ECCO Grant Winner
awarded 20,000 euros
- Dr. Krisztina Gecse and team from the Netherlands
A new ultrasonographic scoring model for stricturing
complications in Crohn’s disease: elastography,
contrast-enhanced and small intestinal contrast
ultrasonography combined (STRICTURE)
